Substituerede amfetaminer
 |

|
| L-ampfetamin | D-ampfetamin |
Substituerede amfetaminer eller amfetaminer er en klasse af kemiske stoffer baseret på amfetamins struktur.[1] Den omfatter alle derivater som er dannet ved at erstatte eller substituere et eller flere brintatomer i amfetamin med substituenter.[1][2][3][4] Forbindelserne i denne gruppe spænder over en række farmakologiske midler, herunder stimulanter, empatogener og hallucinogener og andre.[2] Eksempler på substituerede amfetaminer er amfetamin,[1][2] metamfetamin,[1] efedrin,[1] cathinon,[1] fentermin,[1] mefentermin,[1] bupropion,[1] methoxyfenamin,[1] selegilin,[1] amfepramon,[1] pyrovaleron,[1] MDMA (ecstasy) og DOM (STP).
Nogle af amfetamins substituerede derivater forekommer i naturen, for eksempel i bladene på ledris- og khat-planter, som har været brugt af mennesker i mere end 1000 år for deres farmakologiske virkninger.[1] Amfetamin blev først fremstillet i slutningen af det 19. århundrede. I 1930'erne fandt amfetamin og nogle af dets derivater brug som anti-kongestanter i symptomatisk behandling af forkølelse og også lejlighedsvis som psykoaktive midler. Deres virkninger på centralnervesystemet er forskelligartede, men kan opsummeres ved tre overlappende typer aktivitet: psykoanaleptisk, hallucinogen og empatogen. Forskellige substituerede amfetaminer kan forårsage disse virkninger enten separat eller i kombination.
Eksempler
[redigér | rediger kildetekst]

Amfetaminer er selv en undergruppe af substituerede phenethylamin-forbindelser. Substitution af brintatomer resulterer i en stor klasse af kemiske forbindelser. Typisk reaktioner er substitution med methyl- og nogle gange ethylgrupper på amin- og fenylgrupperne:[5][6][7]
| Stof | Substituenter | Struktur | ||||||
|---|---|---|---|---|---|---|---|---|
| N | α | β | fenylgruppe | |||||
| 2 | 3 | 4 | 5 | |||||
| Phenethylamin | ||||||||
| Amfetamin (α-methylphenylethylamine) | -CH3 | |||||||
| Metamfetamin (N-metylamfetamin) | -CH3 | -CH3 | ||||||
| Fentermin (α-metylamfetamin) | -(CH3)2 | |||||||
| Efedrin | -CH3 | -CH3 | -OH | |||||
| Pseudoefedrin | -CH3 | -CH3 | -OH | |||||
| Cathinon | -CH3 | =O | ||||||
| Metcathinon (efedron) | -CH3 | -CH3 | =O | |||||
| MDA (3,4-methylenedioxyamphetamine) | -CH3 | -O-CH2-O- | ||||||
| MDMA (3,4-metylenedioxymethamfetamin) | -CH3 | -CH3 | -O-CH2-O- | |||||
| MDEA (3,4-metylenedioxy-N-etylamfetamin) | -CH2-CH3 | -CH3 | -O-CH2-O- | |||||
| EDMA (3,4-etylenedioxy-N-metylamfetamin) | -CH3 | -CH3 | -O-CH2-CH2-O- | |||||
| MBDB (N-metyl-1,3-benzodioxolylbutanamin) | -CH3 | -CH2-CH3 | -O-CH2-O- | |||||
| PMA (para-methoxyamfetamin) | -CH3 | -O-CH3 | ||||||
| PMMA (para-metoxymetamfetamin) | -CH3 | -CH3 | -O-CH3 | |||||
| 4-MTA (4-metylthioamfetamin) | -CH3 | -S-CH3 | ||||||
| 3,4-DMA (3,4-dimetoxyamfetamin) | -CH3 | -O-CH3 | -O-CH3 | |||||
| 3,4,5-trimetoxyamfetamin (α-metylmescalin) | -CH3 | -O-CH3 | -O-CH3 | -O-CH3 | ||||
| DOM (2,5-dimethoxy-4-metylamfetamin) | -CH3 | -O-CH3 | -CH3 | -O-CH3 | 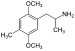
| |||
| DOB (2,5-dimethoxy-4-bromoamfetamin) | -CH3 | -O-CH3 | -Br | -O-CH3 | 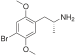
| |||
Referencer
[redigér | rediger kildetekst]- ^ a b c d e f g h i j k l m n Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404-412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ^ a b c Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". Foye's principles of medicinal chemistry (7th udgave). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. s. 646-648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- ^ Lillsunde P, Korte T (marts 1991). "Determination of ring- and N-substituted amphetamines as heptafluorobutyryl derivatives". Forensic Sci. Int. 49 (2): 205-213. doi:10.1016/0379-0738(91)90081-s. PMID 1855720.
- ^ Custodio, Raly James Perez; Botanas, Chrislean Jun; Yoon, Seong Shoon; Peña, June Bryan de la; Peña, Irene Joy dela; Kim, Mikyung; Woo, Taeseon; Seo, Joung-Wook; Jang, Choon-Gon; Kwon, Yong Ho; Kim, Nam Yong (2017-11-01). "Evaluation of the Abuse Potential of Novel Amphetamine Derivatives with Modifications on the Amine (NBNA) and Phenyl (EDA, PMEA, 2-APN) Sites". Biomolecules & Therapeutics (engelsk). 25 (6): 578-585. doi:10.4062/biomolther.2017.141. ISSN 2005-4483. PMC 5685426. PMID 29081089.
- ^ Goldfrank, s. 1125-1127
- ^ Glennon, s. 184-187
- ^ Schatzberg, s. 843
Bibliografi
[redigér | rediger kildetekst]- Ghodse, Hamid (2002). Drugs and Addictive Behaviour. A Guide to Treatment. 3rd Edition. Cambridge University Press. s. 501. ISBN 978-0-511-05844-8.
- Glennon, Richard A. (2008). "Neurobiology of Hallucinogens". The American Psychiatric Publishing textbook of substance abuse treatment. American Psychiatric Publishing. ISBN 978-1-58562-276-4.
- Goldfrank, Lewis R. & Flomenbaum, Neal (2006). Goldfrank's Toxicologic Emergencies, 8th Edition. McGraw Hill. ISBN 978-0-07-147914-1.
- Katzung, Bertram G. (2009). Basic & clinical pharmacology. 11th edition. McGraw-Hill Medical. ISBN 978-0-07-160405-5. (Webside ikke længere tilgængelig)
- Ledgard, Jared (2007). A Laboratory History of Narcotics. Volume 1. Amphetamines and Derivatives. Jared Ledgard. s. 268. ISBN 978-0-615-15694-1.
- Schatzberg, Alan F. & Nemeroff, Charles B. (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. The American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- Snow, Otto (2002). Amphetamine syntheses. Thoth Press. ISBN 978-0-9663128-3-6.
- Veselovskaya NV, Kovalenko AE (2000). Drugs. Properties, effects, pharmacokinetics, metabolism. MA: Triada-X. ISBN 978-5-94497-029-9.
| Infoboks uden skabelon Denne artikel har en infoboks dannet af en tabel eller tilsvarende. |
