Bruger:Christian75/Sandkasse5
pH
[redigér | rediger kildetekst]Skabelon:Lowercase Skabelon:Acids and Bases pH is a measure of the acidity or basicity of a solution. It approximates but is not equal to p[H], the negative logarithm (base 10) of the molar concentration of dissolved hydrogen ions (H+). Crudely, this matches the number of places behind the decimal point, so for example 0.1 molar hydrochloric acid should be near pH 1 and 0.0001 molar HCl should be near pH 4. Pure water is neutral, either a very weak acid or a very weak base (center on the pH scale), giving it a pH of 7, or 0.0000001 M H+.[1] For an aqueous solution to have a higher pH, a base must be dissolved in it, which binds away many of these rare hydrogen ions. Hydrogen ions in water can be written simply as H+ or as hydronium (H3O+) or higher species (e.g. H9O4+) to account for solvation, but all describe the same entity.
However, pH is not precisely p[H], but takes into account an activity factor, which represents the tendency of hydrogen ions to interact with other components of the solution, which affects among other things the electrical potential read using a pH meter. As a result, pH can be affected by the ionic strength of a solution - for example, the pH of a 0.05 M potassium hydrogen phthalate solution can vary by as much as 0.5 pH units as a function of added potassium chloride, even though the added salt is neither acidic nor basic.[2]
Unfortunately, hydrogen ion activity coefficients cannot be measured directly by any thermodynamically sound method, so they are based on theoretical calculations. Therefore the pH scale is defined in practice as traceable to a set of standard solutions whose pH is established by international agreement.[3] Primary pH standard values are determined by the Harned cell, a hydrogen gas electrode, using the Bates-Guggenheim Convention.
Pure water is said to be neutral, with a pH close to 7.0 at 25 °C (77 °F). Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are said to be basic or alkaline. pH measurements are important in medicine, biology, chemistry, food science, environmental science, oceanography and many other applications.
History
[redigér | rediger kildetekst]The concept of p[H] was first introduced by Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909[4] [5] and revised to the modern pH in 1924 after it became apparent that electromotive force in cells depended on activity rather than concentration of hydrogen ions.[2] It is unknown what the exact definition of p is. Some references suggest the p stands for “Power”,[6] others refer to the German word “Potenz” (meaning power in German),[7] still others refer to “potential”. Jens Norby published a paper in 2000 arguing that p is a constant and stands for “negative logarithm”;[8] which has also been used in other works.[9] H then stands for Hydrogen. According to the Carlsberg Foundation pH stands for "power of hydrogen".[6] Other suggestions that have surfaced over the years are that the p stands for puissance (also meaning power but then the Carlsberg Laboratory was French speaking) or that pH stands for the Latin terms pondus Hydrogenii or potentia hydrogenii. It is also suggested that Sørensen used the letters p and q (commonly paired letters in mathematics) simply to label the test solution (p) and the reference solution (q).[10]
Definitions
[redigér | rediger kildetekst]Mathematical Definition
[redigér | rediger kildetekst]pH is defined as minus the decimal logarithm of the hydrogen ion activity in a solution.[11] By virtue of its logarithmic nature, pH is a dimensionless quantity.
where aH is the (dimensionless) activity of hydrogen ions. The reason for this definition is that aH is a property of a single ion, which can only be measured experimentally by means of an ion-selective electrode which responds, according to the Nernst equation, to hydrogen ion activity. pH is commonly measured by means of a combined glass electrode, which measures the potential difference, or electromotive force, E, between an electrode sensitive to the hydrogen ion activity and a reference electrode, such as a calomel electrode or a silver chloride electrode. The combined glass electrode ideally follows the Nernst equation:
where E is a measured potential , E0 is the standard electrode potential, that is, the electode potential for the standard state in which the activity is one. R is the gas constant, T is the temperature in kelvins, F is the Faraday constant and n is the number of electrons transferred, one in this instance. The electrode potential, E, is proportional to the logarithm of the hydrogen ion activity.
This definition, by itself, is wholly impractical, because the hydrogen ion activity is the product of the concentration and an activity coefficient. The single-ion activity coefficient of the hydrogen ion is a quantity which cannot be measured experimentally. To get around this difficulty, the electrode is calibrated in terms of solutions of known activity.
The operational definition of pH is officially defined by International Standard ISO 31-8 as follows: [12] For a solution X, first measure the electromotive force EX of the galvanic cell
- reference electrode | concentrated solution of KCl || solution X | H2 | Pt
and then also measure the electromotive force ES of a galvanic cell that differs from the above one only by the replacement of the solution X of unknown pH, pH(X), by a solution S of a known standard pH, pH(S). The pH of X is then
The difference between the pH of solution X and the pH of the standard solution depends only on the difference between two measured potentials. Thus, pH is obtained from a potential measured with an electrode calibrated against one or more pH standards; a pH meter setting is adjusted such that the meter reading for a solution of a standard is equal to the value pH(S). Values pH(S) for a range of standard solutions S, along with further details, are given in the IUPAC recommendations.[13] The standard solutions are often described as standard buffer solution. In practice, it is better to use two or more standard buffers to allow for small deviations from Nernst-law ideality in real electrodes. Note that because the temperature occurs in the defining equations, the pH of a solution is temperature-dependent.
Measurement of extremely low pH values, such as some very acidic mine waters,[14] requires special procedures. Calibration of the electrode in such cases can be done with standard solutions of concentrated sulfuric acid, whose pH values can be calculated with using Pitzer parameters to calculate activity coefficients.[15]
pH is an example of an acidity function. Hydrogen ion concentrations can be measured in non-aqueous solvents, but this leads, in effect, to a different acidity function, because the standard state for a non-aqueous solvent is different from the standard state for water. Superacids are a class of non-aqueous acids for which the Hammett acidity function, H0, has been developed.
p[H]
[redigér | rediger kildetekst]This was the original definition of Sørensen, [6] which was superseded in favour of pH.[hvornår?] However, it is possible to measure the concentration of hydrogen ions directly, if the electrode is calibrated in terms of hydrogen ion concentrations. One way to do this, which has been used extensively, is to titrate a solution of known concentration of a strong acid with a solution of known concentration of strong alkali in the presence of a relatively high concentration of background electrolyte. Since the concentrations of acid and alkali are known it is easy to calculate the concentration of hydrogen ions so that the measured potential can be correlated with concentrations. The calibration is usually carried out using a Gran plot.[16] The calibration yieds a value for the standard electrode potential, E0, and a slope factor, f, so that the Nernst equation in the form
can be used to derive hydrogen ion concentrations from experimental measurements of E. The slope factor is usually slightly less than one. A slope factor of less than 0.95 indicates that the electrode is not functioning correctly. The presence of background electrolyte ensures that the hydrogen ion activity coefficient is effectively constant during the titration. As it is constant its value can be set to one by defining the standard state as being the solution containing the background electrolyte. Thus, the effect of using this procedure is to make activity equal to the numerical value of concentration.
The difference between p[H] and pH is quite small. It has been stated[17] that pH = p[H] + 0.04. Unfortunately it is common practice to use the term "pH" for both types of measurement.
pOH
[redigér | rediger kildetekst]pOH is sometimes used as a measure of the concentration of hydroxide ions, OH−, or alkalinity. pOH is not measured independently, but is derived from pH. The concentration of hydroxide ions in water is related to the concentration of hydrogen ions by
- [OH−] = KW /[H+]
where KW is the self-ionisation constant of water. Taking cologarithms
- pOH = pKW − pH.
So, at room temperature pOH ≈ 14 − pH. However this relationship is not strictly valid in other circumstances, such as in measurements of soil alkalinity.
Applications
[redigér | rediger kildetekst]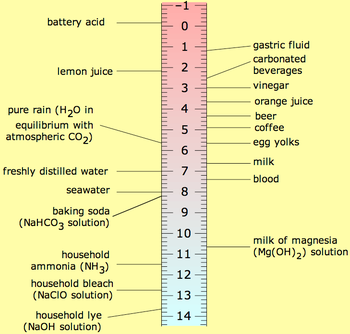
Pure water has a pH around 7; the exact values depends on the temperature. When an acid is dissolved in water the pH will be less than 7 and when a base, or alkali is dissolved in water the pH will be greater than 7. A solution of a strong acid, such as hydrochloric acid, at concentration 1 mol dm−3 has a pH of 0. A solution of a strong alkali, such as sodium hydroxide, at concentration 1 mol dm−3 has a pH of 14. Thus, measured pH values will mostly lie in the range 0 to 14. Since pH is a logarithmic scale a difference of one pH unit is equivalent to a ten-fold difference in hydrogen ion concentration.
Because the glass electrode (and other ion selective electrodes) responds to activity, the electrode should be calibrated in a medium similar to the one being investigated. For instance, if one wishes to measure the pH of a seawater sample, the electrode should be calibrated in a solution resembling seawater in its chemical composition, as detailed below.
An approximate measure of pH may be obtained by using a pH indicator. A pH indicator is a substance that changes colour around a particular pH value. It is a weak acid or weak base and the colour change occurs around 1 pH unit either side of its acid dissociation constant, or pKa, value. For example, the naturally occurring indicator litmus is red in acidic solutions (pH<7) and blue in alkaline (pH>7) solutions. Universal indicator consists of a mixture of indicators such that there is a continuous colour change from about pH 2 to pH 10. Universal indicator paper is simple paper that has been impregnated with universal indicator.
| Indicator | Low pH color | Transition pH range | High pH color |
|---|---|---|---|
| Thymol blue (first transition) | red | 1.2–2.8 | orange |
| Methyl red | red | 4.4–6.2 | yellow |
| Bromothymol blue | yellow | 6.0–7.6 | blue |
| Thymol blue (second transition) | yellow | 8.0–9.6 | blue |
| Phenolphthalein | colorless | 8.3–10.0 | purple |
A solution whose pH is 7 is said to be neutral, that is, it is neither acidic nor basic. Water is subject to a self-ionisation process.
- H2O Skabelon:Eqm H+ + OH−
The dissociation constant, KW, has a value of about 10-14, so in neutral solution of a salt both the hydrogen ion concentration and hydroxide ion concentration are about 10-7 mol dm-3. The pH of pure water decreases with increasing temperatures. For example, the pH of pure water at 50 °C is 6.55. Note, however, that water that has been exposed to air is mildly acidic. This is because water absorbs carbon dioxide from the air, which is then slowly converted into carbonic acid, which dissociates to liberate hydrogen ions:
- CO2 + H2O Skabelon:Eqm H2CO3 Skabelon:Eqm HCO3− + H+
Calculation of pH for weak and strong acids
[redigér | rediger kildetekst]In the case of a strong acid, there is complete dissociation, so the pH is simply equal to minus the logarithm of the acid concentration. For example, a 0.01 molar solution of hydrochloric acid has a pH of −log(0.01), that is, pH = 2.
The pH of a solution of a weak acid may be calculated by means of an ICE table. For acids with a pKa value greater than about 2,
- pH = ½ ( pKa − log c0),
where c0 is the concentration of the acid. This is equivalent to Burrows' weak acid pH equation
A more general method is as follows. Consider the case of dissolving a weak acid, HA, in water. First write down the equilibrium expression.
- HA A− + H+
The equilibrium constant for this reaction is specified by
where [] indicates a concentration. The analytical concentration of the two reagents, CA for [A−] and CH for [H+] must be equal to the sum of concentrations of those species that contain the reagents. CH is the concentration of added mineral acid.
- CA = [A−] + Ka[A−][H+]
- CH = [H+] + Ka[A−][H+]
From the first equation
Substitution of this expression into the second equation gives
This simplifies to a quadratic equation in the hydrogen ion concentration
Solution of this equation gives [H+] and hence pH.
This method can also be used for polyprotic acids. For example, for the diprotic acid oxalic acid, writing A2− for the oxalate ion,
- CA = [A2−] + β1[A2−][H+] + β2[A2−][H+]2
- CH = [H+] + β1[A2−][H+] + 2β2[A2−][H+]2
where β1 and β2 are cumulative protonation constants. Following the same procedure of substituting from the first equation into the second, a cubic equation in [H+] results. In general, the degree of the equation is one more than the number of ionisable protons. The solution of these equations can be obtained relatively easily with the aid of a spreadsheet such as EXCEL or Origin. The pH always has an amount of fractional figures equal to the amount of significant figures of the concentration.
pH in nature
[redigér | rediger kildetekst]
pH-dependent plant pigments that can be used as pH indicators occur in many plants, including hibiscus, marigold, red cabbage (anthocyanin),[18] and red wine.
Seawater
[redigér | rediger kildetekst]The pH of seawater is very important and there is evidence for ocean acidification. Distinct pH scales exist depending on the method of determination.[19]
- NBS Scale, denoted pHNBS. This scale is useful for pH determinations by galvanic cells calibrated with NIST standards. Unfortunately, the ionic strength of the standard buffer solutions are much lower(~0.1 M) than that of seawater (~0.7 M). Consequently, a strong liquid junction potential perturbation leaves the pHNBS scale not recommended for use with seawater pH determinations.
- The total scale, denoted pHT. A set of buffers based on artificial seawater was developed.[20] This pH scale is referred to as the total scale, denoted by pHT. The total scale was defined using a medium containing sulfate ions, which are subject to the proton absorbing equilibrium H+ + SO42− Skabelon:Eqm HSO4−.
- The free scale, denoted pHF. This scale omits the effect of sulfate ions and focuses solely on [H+]F, in principle making it a simpler representation of hydrogen ion concentration. Analytically, only [H+]T can be determined,[21] therefore, [H+]F must be estimated using the [SO42−] and the dissociation constant constant of HSO4−. The utility of this scale is limited by the complexity of the calculations. pH values measured on the free scale differ by up to 0.12 pH units from both the total and seawater scales.
- The seawater scale, denoted pHSWS . Lastly, the seawater scale takes into account of the fact that hydrogen fluoride is a weak acid, H+ + F− Skabelon:Eqm HF. However, the concentration of sulfate ions is about 400 times larger than the concentration of fluoride, so the difference between the total and seawater scales is very small.
Living systems
[redigér | rediger kildetekst]| Compartment | pH |
|---|---|
| Gastric acid | 0.7 |
| Lysosomes | 4.5 |
| Granules of chromaffin cells | 5.5 |
| Human skin | 5.5 |
| Urine | 6.0 |
| Neutral H2O at 37 °C | 6.81 |
| Cytosol | 7.2 |
| Cerebrospinal fluid (CSF) | 7.3 |
| Blood | 7.34–7.45 |
| Mitochondrial matrix | 7.5 |
| Pancreas secretions | 8.1 |

The pH of different cellular compartments, body fluids, and organs is usually tightly regulated in a process called acid-base homeostasis.
The pH of blood is usually slightly basic with a value of pH 7.4. This value is often referred to as physiological pH in biology and medicine.
Plaque can create a local acidic environment that can result in tooth decay by demineralisation.
Enzymes and other proteins have an optimum pH range and can become inactivated or denatured outside this range.
The most common disorder in acid-base homeostasis is acidosis, which means an acid overload in the body, generally defined by pH falling below 7.35.
In the body, pH can be estimated from known base excess (be) and bicarbonate concentration (HCO3) by the following equation:[25]
See also
[redigér | rediger kildetekst]References
[redigér | rediger kildetekst]- ^ "General Chemistry Handouts, Pomona College: How to Solve Equilibrium Problems: An Approach Based on Stoichiometry Part II, Acid-Base Reactions".
{{cite web}}: line feed character i|title=på position 114 (hjælp) - ^ a b original reference requires subscription Isaac Feldman (1956). "Use and Abuse of pH measurements". - cited and applied in publicly accessible "13C and 170 NMR Binding Constant Studies of Uranyl Carbonate Complexes in Near-Neutral Aqueous Solution Yucca Mountain Project Milestone Report 3351".
{{cite web}}: Manglende eller tom|url=(hjælp); Ukendt parameter|http://www.osti.gov/bridge/purl.cover.jsp?purl=ignoreret (hjælp) - - ^ "The Measurement of pH - Definition, Standards and Procedures] – Report of the Working Party on pH, IUPAC Provisional Recommendation" (PDF). 2001. A proposal to revise the current IUPAC 1985 and ISO 31-8 definition of pH.
- ^ 319 Sorensen, S. P. L., Enzymstudien. II, Ueber die Messung und die Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen, Biochem. Zeitschr., 1909, vol. 21, pp. 131-304.
- ^ Two other publications appeared in 1909 one in French and one in Danisch
- ^ a b c Carlsberg Group Company History Page, http://www.carlsberggroup.com/Company/Research/Pages/pHValue.aspx
- ^ University of Waterloo - The pH Scale, http://www.science.uwaterloo.ca/~cchieh/cact/c123/ph.html
- ^ Nørby, Jens. 2000. The origin and the meaning of the little p in pH. Trends in the Biochemical Sciences 25:36-37., http://download.cell.com/trends/biochemical-sciences/pdf/PIIS0968000499015170.pdf
- ^ Fundamentals of Analytical Toxicology, http://books.google.com.br/books?id=LBag6XlAJY0C
- ^ One-Hundred Years of pH Rollie J. Myers Journal of Chemical Education Vol. 87 No. 1 January 2010
- ^ "pH". IUPAC Goldbook.
- ^ Quantities and units – Part 8: Physical chemistry and molecular physics, Annex C (normative): pH. International Organization for Standardization, 1992.
- ^ Definitions of pH scales, standard reference values, measurement of pH, and related terminology. Pure Appl. Chem. (1985), 57, pp 531–542.
- ^ Nordstrom, DK et al. (2000) Negative pH and extremely acidic mine waters from Iron Mountain California. Environ Sci Technol,34, 254-258.
- ^ Zemaitis, J.F. (1986). Handbook of Aqueous Electrolyte Thermodynamics: Theory & Application. Wiley. ISBN 978-0-8169-0350-4.
{{cite book}}: Ukendt parameter|coauthors=ignoreret (|author=foreslået) (hjælp) Chapter 4 - ^ Rossotti, F.J.C. (1965). "Potentiometric titrations using Gran plots: A textbook omission". J. Chem. Ed. 42: 375-378.
{{cite journal}}: Ukendt parameter|coauthors=ignoreret (|author=foreslået) (hjælp) - ^ Skabelon:VogelQuantitative Section 13.23, "Determination of pH"
- ^ chemistry.about.com
- ^ Zeebe, R.E. (2001). CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier. ISBN 0 444 50946 1.
{{cite book}}: Ukendt parameter|coauthors=ignoreret (|author=foreslået) (hjælp) - ^ Hansson, I (1973). "A new set of pH-scales and standard buffers for seawater". Deep Sea Research. 20: 479-491. doi:10.1016/0011-7471(73)90101-0.
- ^ Dickson, A. G. (1984). "pH scales and proton-transfer reactions in saline media such as sea water". Geochim. Cosmochim. Acta. 48: 2299-2308. doi:10.1016/0016-7037(84)90225-4.
- ^ Boron, Walter, F. (2004). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3.
{{cite book}}: Ukendt parameter|coauthors=ignoreret (|author=foreslået) (hjælp) - ^ Answers.com Medical Encyclopedia: Metabolic Acidosis: Causes and symptoms By Altha Roberts Edgren. Retrieved on April 13, 2009
- ^ Symptoms mentioned in both metabolic and respiratory acidosis from the following two references:
- Wrongdiagnosis.com > Symptoms of Metabolic Acidosis Retrieved on April 13, 2009
- Wrongdiagnosis.com > Symptoms of Respiratory acidosis Retrieved on April 13, 2009 - ^ Medical Calculators > Calculated Bicarbonate & Base Excess teven Pon, MD, Weill Medical College of Cornell University
External links
[redigér | rediger kildetekst][[Category:Acid-base chemistry]] [[Category:Equilibrium chemistry]] [[Category:Units of measure]] [[Category:Water quality indicators]]
ICE table
[redigér | rediger kildetekst]An ICE table or ICE chart is a tabular system of keeping track of changing concentrations in an equilibrium reaction. ICE stands for "Initial, Change, Equilibrium". It is used in chemistry to keep track of the changes in amount of substance of the reactants and also organize a set of conditions that one wants to solve with. Occasionally, the chemical reaction is written at the top of the table, resulting in a RICE table.
Example
[redigér | rediger kildetekst]To illustrate the processes, consider the case of dissolving a weak acid, HA, in water. How can the pH be calculated? First write down the equilibrium expression.
- HA A- + H+
The columns of the table correspond to the three species in equilibrium.
| [HA] | [A-] | [H+] | |
| I | c0 | 0 | 0 |
| C | -x | +x | +x |
| E | c0 - x | x | x |
The first row, labelled I, has the initial conditions: the concentration of acid is c0 and it is initially undissociated so the concentrations of A- and H+ are zero.
The second row, labelled C, specifies that when the acid dissociates, its concentration changes by an amount -x and the concentrations of A- and H+ both change by an amount +x. This follows from the equilibrium expression. The columns which have initial values of zero will always be added to (augmented).
The third row, labelled E, is the sum of the first two rows and shows the concentrations at equilibrium.
It can be seen from the table that at equilibrium [H+] = x.
To find x the equilibrium constant must be specified.
Substitute the concentrations with the values found in the last row of the ICE table.
With specific values for c0 and Ka this equation can be solved for x. Assuming[1] that pH = -log10[H+] the pH can be calculated as pH = -log10x.
When the degree of dissociation is quite small, c0 >> x and the expression simplifies to
and pH= 1/2( pKa - log c0). This approximate expression is good for pKa values larger than about 2.
References
[redigér | rediger kildetekst]- ^ Strictly speaking pH is equal to -log10{H+} where {H+} is the activity of the hydrogen ion. In dilute solution concentration is almost equal to activity
[[Category:Equilibrium chemistry]] [[Category:Physical chemistry]] <!--[[en:ICE table]] [[vi:Bảng Bắt đầu-Phản ứng-Cân bằng]]
Industri
[redigér | rediger kildetekst]Industri refererer til produktionen af økonomiske goder (enten materielle eller som service) indenfor en økonomi. Disse inddeles ofte i op til fem økonomiske sektorer: Den primære sektor indeholder råstofindvinding som fx minedrift, landbrug og fiskeri. Den sekundære sektor (fremstillingsindustrien) indeholder raffinaderi, konstruktion og fabrikation. Den tertiære har med handel, service samt distrubution af de producerede goder (transport). Den kvaternære sektor er en relativ ny vidensindustri som fokusrer på teknologisk forskning, design og udvikling, som fx computer programmering og biokemi. En femte sektor er blevet foreslået til at indeholde nonprofit aktiviteter (som fx at skrive for wikipedia)
The economy is also broadly separated into public sector and private sector, with industry generally categorized as private. Industries are also any business or manufacturing.
Danske forhold
[redigér | rediger kildetekst]Industri (branche) - betegner en gren af det økonomiske liv ("bilindustrien", "tobaksindustrien"). Kommer fra engelsk (industry), men industri er nu blevet en integreret del af dansk sprog.
Industri (masseproduktion) - i modsætning til håndværksproduktion. Danmarks Statistiks industristatistik baserer sig på denne betydning, idet denne omfatter virksomheder med mindst 10 ansatte. Fra disse har man (gennemsnitligt) mere pålidelige indberetninger end fra de mindre håndværksvirksomheder.
- Industri (fremstillingsindustri) - betegnelse for den sekundære sektor, ved opdeling af hele det økonomiske liv i tre (eller fire) sektorer: Den tertiære sektor er handel, service og transport. Det kvartære sektor er man begyndt at tale om i nyere tid, og omfatter fremstilling og forarbedning af viden.




![{\displaystyle E=E^{0}+f{\frac {RT}{nF}}\log _{e}[{\mbox{H}}^{+}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/85584d1ffca5ec688288b919f844534e2658c011)


![{\displaystyle K_{\text{a}}=\mathrm {\frac {[A^{-}][H^{+}]}{[HA]}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/fa9f960c87ed3dbf034fd4671dcb775e4efb7072)
![{\displaystyle \mathrm {[A^{-}]={\frac {{\mathit {C}}_{A}}{1+{\mathit {K}}_{a}[H^{+}]}}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/13e1542f3acc7e7b0ab7b4558de2318e0ba175f3)
![{\displaystyle \mathrm {{\mathit {C}}_{H}=[H^{+}]+{\frac {{\mathit {K}}_{a}{\mathit {C}}_{A}[H^{+}]}{1+{\mathit {K}}_{a}[H^{+}]}}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/3224ecf8d489051d95b8b9a707afcd59eb9c52ab)
![{\displaystyle \mathrm {{\mathit {K}}_{a}[H^{+}]^{2}+{\bigg (}1+({\mathit {C}}_{A}-{\mathit {C}}_{H}){\mathit {K}}_{a}{\bigg )}[H^{+}]-{\mathit {C}}_{H}=0} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/addbd36ee8d6580bcb3ee1bfacab7aef46d02fb2)


![{\displaystyle K_{a}={\frac {[H^{+}][A^{-}]}{[HA]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/83ee86c6746a584bd7b209324db405b0563af917)

